Lithium-ion batteries (LIBs) are widely used in portable electronic devices such as smartphones and laptops because they are lightweight and have high charge/discharge efficiency. They can also store a large amount of electricity and are used in electric vehicles and energy storage systems.
The electrolyte used in commonly used LIBs is an organic solvent-based liquid. This has the advantage of high conductivity, but the disadvantages of leakage, ignition due to high temperatures, and performance degradation below freezing. To overcome these drawbacks, various research institutes are actively researching “solid-state batteries,” which use solid electrolytes instead of liquids ones, with the aim of commercializing them.
In addition to eliminating the risk of leakage, solid electrolytes are resistant to temperature changes, so they have many advantages, such as high safety, low deterioration, and a long lifespan. Another advantage unique to solid-state batteries is that the structure and shape can be freely changed, which allows for a high degree of design freedom and makes them easy to apply to products. Currently, various manufacturers are actively developing solid-state batteries with the aim of introducing them into electric vehicles, including the development of innovative batteries that can run over 1,000 km on a charge of less than 10 minutes.
The performance of solid-state batteries depends on the crystal structure of the solid electrolyte’s ionic conductivity properties and thermal and chemical stability, so analysis of this crystal structure is vital. Rigaku is contributing to the research and development of solid electrolytes by making full use of various analytical methods, including X-ray diffraction.
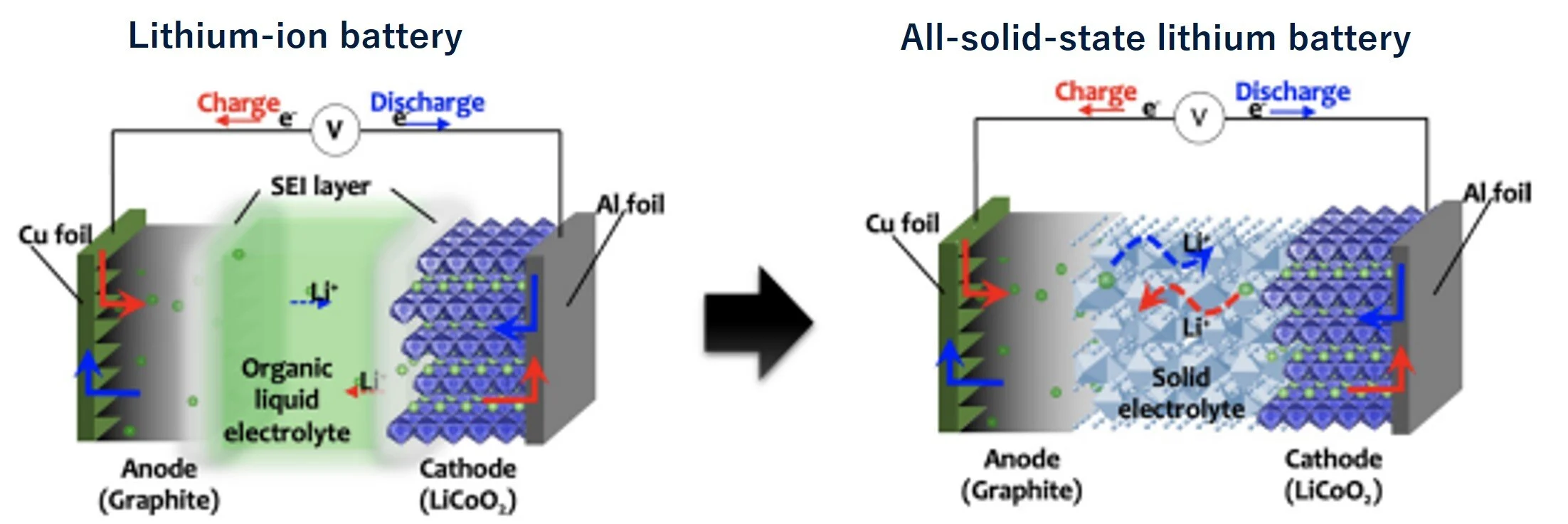
Schematic diagram of the constituent materials and reactions of lithium-ion batteries and all-solid-state lithium
Kota Suzuki, Masaaki Hirayama, Ryoji Kanno, Rigaku Journal 52(1), (2021), 1-8
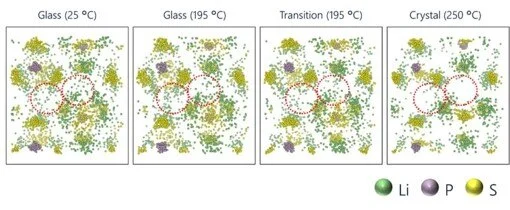
Atomic distribution in the unit cell of the all-solid-state electrolyte Li₃PS₄ at each measurement temperature
M. Yoshimoto, T. Kimura, A. Sakuda, C. Hotehama, Y. Shiramata, A. Hayashi, K. Omote, Solid State Ionics, 401 (2023), 116361 (8 pp).
When Li₃PS₄ is heated and the atomic distribution is examined in each of the glass, transition, and crystalline states, it is suggested that Li is diffused in the glass state resulting in high electrical conductivity, while Li is aggregated in the crystalline state resulting in low electrical conductivity.
This trend is similar to that seen in the experimental results for electrical conductivity.
